Working in the vanEngelsdorp lab this past semester, I had the opportunity to help develop a procedure for extracting lipids from honey bees (the significance of which you can read about here). The lab courses I’ve taken so far have all required me to follow procedures like a recipe, which is good way to develop your lab technique, but leaves no room for creativity. Along with my lipid extraction compatriots Sue Boo and Todd Waters, I found out that developing a procedure is all about creativity. Although we were fortunate to gain insight from many of the lab’s research assistants, we were also given the freedom to explore different methods and to follow our intuition.
Our first step was to review the literature on lipid extraction from animal tissues. We quickly found that the methods sections of most scientific papers are not written to be immediately reproducible. It became clear that lipid extraction is such a basic process that most papers barely mentioned their extraction methods.
One lipid extraction method we considered was a Soxhlet extraction, which requires a Soxhlet extractor (Figure 1) and allows the continuous re-use of a fixed amount of solvent. Go down to the bottom of this post if you’re interested in learning how the Soxhlet extractor works.* Although we were enthralled by the sheer ingenuity of this piece of equipment, it was clear that lipid extraction could be performed with a much simpler procedure using common glassware.
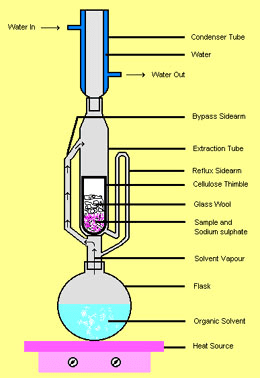
Image source: http://whale.wheelock.edu/bwcontaminants/analysis.html
After seeing the “Folch method” mentioned several times in scientific papers, we found Folch’s original methods paper, which became our main reference and gave us the basic outline of our protocol: a) homogenize the tissue in a nonpolar, lipophilic solvent, b) add a polar solvent like water to create a two-phase solution, and c) extract the nonpolar phase and remove the solvent (1).
The Folch method uses a 2:1 chloroform-methanol mixture as the lipid solvent. Although we would be working under a fume hood, we didn’t want to unnecessarily expose the entire lab to something as nasty as chloroform. After consulting with Dr. Frederick Khachik, an organic chemistry professor and director of the Organic I lab course, we decided to use dichloromethane instead. Furthermore, dichloromethane has been shown to be as effective as chloroform at extracting lipids in a study using plasma and liver tissue (2).
As soon as we began performing our extractions, we encountered difficulties during the homogenization step. The bees we’ve used for our extractions had been stored whole in a freezer. We were then freezing them in liquid nitrogen so they wouldn’t thaw as we crushed them in the mortar and pestle (Figure 2). Freezing in liquid nitrogen helped extend the amount of time the bees remained frozen, but as soon as they thawed, they left a large amount of residue in the mortar which was hard to recover. We then started crushing the bees inside the liquid nitrogen extraction bag using the pestle and a stone block.

Often, in the midst of doing an extraction, we’d have an idea that would transform the process. While following our written procedure was crucial, at times breaking out of it was also necessary. Our best homogenization of the bees resulted from freezing them in liquid nitrogen, crushing them in the bag until they thawed, re-freezing them, crushing them again until they thawed, and so on until we had a fairly fine bee powder. We then scraped this powder into a beaker with dichloromethane and a magnetic stirbar, and let the powder thaw into the solvent while being continuously stirred on a magnetic stir plate (Figure 3). Many of our greatest improvements came from this on-the-spot insight and wondering about what would happen if we tried some other way. Sometimes these ideas come from a place of true understanding, other times simply from a vague inkling of what might result, but you test them, and whatever works prevails.

Above all, this semester I learned that science is inherently a creative process. This creativity is not restricted to preeminent scientists of the day or geniuses like Franz von Soxhlet; it’s needed at all levels. I am reminded how the biologist François Jacob described the process of discovering the operon:
“Our breakthrough was the result of ‘night science’: a stumbling, wandering exploration of the natural world that relies on intuition as much as it does on the cold, orderly logic of ‘day science’.” (3)
References
- Folch, J., Lees, M., & Sloane Stanley, G. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem., 226:497-509.
- Christie, W. (1993). Preparation of lipid extracts from tissues. Advances in Lipid Methodology, 2:195-213. Dundee, Scotland: Oily Press.
- Jacob, F. (1988). The statue within: an autobiography. Long Island, NY: Cold Spring Harbor Laboratory Press.
*How Soxhlet extraction works: The solvent is placed in the round bottom flask; as it’s heated, the vapor travels up the large bypass sidearm (pictured on the left in Figure 1), condenses in the condenser, and drops into the extraction chamber. There it encounters the tissue sample and dissolves the lipids. As liquid builds up in the extraction chamber, it is pushed up the small siphon side-arm tube, until it tips over the top curve of the siphon tube, pulling the liquid in the extraction chamber down into the round bottom flask. At this point, solvent in the flask will continue to vaporize, condense into the extraction chamber, and get pulled back into the flask, carrying more and more lipids from the tissue sample with it each time. You can keep the reflux going for as long as you wish; it will continuously re-use the solvent and concentrate the lipids in one place.